Energy storage doesn’t usually make headlines, yet it quietly decides how far an electric car can drive, how reliable solar power can be at night, and even how thin our phones can become. For years, engineers have tried to move beyond lithium-ion batteries, and now the unusual sulfur chemistry battery is drawing serious attention.

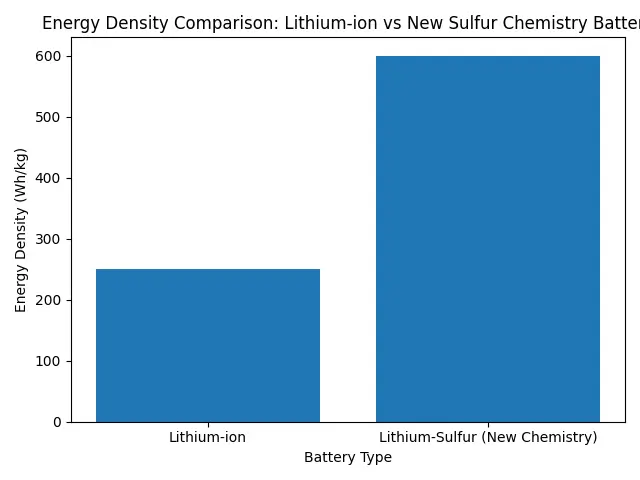
The unusual sulfur chemistry battery offers a rare possibility in modern technology not a small upgrade, but a genuine leap in how much energy a battery can hold. Lithium-ion cells have served us well since the 1990s. They powered the smartphone era and helped launch electric vehicles into the mainstream. But today the demands are very different. Electric trucks need longer ranges, renewable grids need days of storage instead of hours, and manufacturers want cheaper materials that don’t rely on scarce metals. Researchers believe a new sulfur-based design could be the answer, and early lab results suggest it might finally solve problems that have frustrated battery scientists for decades.
The unusual sulfur chemistry battery is essentially a redesigned lithium-sulfur battery, but with a critical difference: scientists now control the reactions inside the cell instead of trying to contain the damage they cause. In earlier lithium-sulfur designs, sulfur dissolved during operation and gradually destroyed the battery. This new approach changes sulfur’s behavior at a microscopic level. Researchers built a structured material that holds sulfur molecules in place during charging and discharging. Instead of drifting through the battery and degrading performance, the sulfur follows a stable reaction pathway. This dramatically improves lifespan while keeping sulfur’s biggest advantage extremely high energy density. In practical terms, it means a battery can store more electricity without becoming larger or heavier, which is exactly what electric vehicles and renewable energy systems need.
Table of Contents
Researchers Develop a Powerful New Battery
| Key Feature | Details |
|---|---|
| Battery Type | Advanced lithium-sulfur battery |
| Main Innovation | Controlled sulfur reaction pathway |
| Core Material | Sulfur inside conductive host structure |
| Energy Density | Up to two or three times lithium-ion |
| Main Problem Solved | Polysulfide shuttle effect |
| Stability | Improved charge cycle lifespan |
| Cost Advantage | Uses abundant low-cost sulfur |
| Environmental Benefit | Less reliance on cobalt and nickel |
| Possible Applications | EVs, aircraft, grid storage, electronics |
| Current Stage | Laboratory development progressing toward commercialization |
Why Sulfur Attracts Battery Researchers
- Scientists have studied sulfur for years because, on paper, it looks almost perfect. It can store far more energy than the metal oxides used in lithium-ion batteries. A working unusual sulfur chemistry battery could power a vehicle far longer without increasing battery weight, which is a major barrier to adoption for heavy vehicles and aircraft.
- There is also an economic reason. Materials like cobalt and nickel are expensive and come from limited mining regions. Supply disruptions can quickly increase battery prices. Sulfur, by contrast, is abundant and often produced as a by-product of industrial refining. That makes it cheap and widely available.
- The problem has always been instability. When sulfur reacts with lithium, it forms compounds called polysulfides. These compounds dissolve in the electrolyte and migrate across the battery. Over time, the battery loses its active material and stops holding a charge. This issue prevented lithium-sulfur technology from leaving the lab for many years.
The Unusual Sulfur Chemistry Explained
The new unusual sulfur chemistry battery solves the instability problem by changing how the chemical reactions happen rather than trying to block them entirely. Researchers created a host material a conductive framework that traps sulfur compounds during operation.
Inside the battery, the process works like this:
- Sulfur reacts with lithium during discharge.
- Intermediate compounds form but remain confined within the structure.
- When the battery recharges, the reaction reverses and sulfur reforms.
Because these compounds can’t escape, the battery no longer loses active material. Earlier batteries allowed the reaction products to move freely, damaging the lithium electrode and rapidly reducing capacity. The new system shortens the chemical pathway and stabilizes the molecules involved. This controlled reaction sequence is what makes the chemistry unusual. Instead of long unstable molecular chains, the reactions form shorter, stable ones. The battery becomes efficient and repeatable exactly what a commercial product requires.
Improved Performance and Cycle Life
Performance is the real reason engineers are excited about the unusual sulfur chemistry battery. Lithium-ion technology is approaching its theoretical maximum energy density, meaning future improvements will be small. Lithium-sulfur still has significant room for improvement. The new design offers three clear advantages.
- First, higher energy density. A battery can store far more electricity for the same weight. Electric cars could travel hundreds of additional kilometers without larger battery packs.
- Second, longer cycle life. Stabilized reactions mean the battery survives many more charge cycles than earlier prototypes.
- Third, better efficiency. Less energy is lost as heat, which can improve charging speed and safety.
Researchers also observed more stable voltage behavior during operation, which is important for sensitive electronics and power systems.
Potential Applications
Electric Vehicles
For electric cars, range anxiety remains a major concern. Drivers worry about running out of power between charging stations. A successful unusual sulfur chemistry battery could significantly extend range while reducing battery weight. Lighter vehicles also improve efficiency, meaning less electricity is needed per kilometer.
Aviation
Electric aviation has always struggled with battery weight. Aircraft require extremely high energy per kilogram, and lithium-ion batteries are simply too heavy for long-distance flights. Sulfur batteries offer one of the few realistic solutions for electric regional aircraft and cargo drones.
Renewable Energy Storage
Solar panels produce electricity during the day, while wind turbines generate power unpredictably. Large stationary battery systems are essential for grid stability. A grid-scale unusual sulfur chemistry battery installation could store excess renewable energy and release it overnight, helping cities rely less on fossil fuels.
Consumer Electronics
Phones and laptops could benefit as well. Devices might run for days instead of hours. Reduced heat generation would also improve device safety and battery longevity.
Safety And Cost Advantages
- One overlooked benefit of sulfur batteries is environmental impact. Many lithium-ion batteries rely on cobalt mining, which raises ethical and environmental concerns. Sulfur is widely available and less damaging to source.
- Cost is another major factor. Because sulfur is inexpensive, future battery packs could be cheaper to produce once manufacturing scales up. Since batteries represent a large portion of electric vehicle cost, cheaper energy storage could make EVs more affordable.
- Safety may improve too. Controlled chemical reactions produce less heat buildup. Thermal runaway the chain reaction responsible for most battery fires becomes less likely when reactions are stable.
Challenges That Remain
Despite promising results, the unusual sulfur chemistry battery is not yet ready for mass production. Laboratory success must translate into real-world reliability.
Several engineering hurdles remain:
- Manufacturing methods must be scaled efficiently.
- Lithium metal electrodes require protective designs.
- Long-term durability must be proven over thousands of cycles.
- Integration with existing charging systems must be tested.
These are complex challenges, but importantly they are engineering problems, not fundamental scientific barriers.
What This Means for The Future of Batteries
- Battery development often progresses slowly until a key discovery changes direction. This research suggests lithium-ion may not be the final stage of energy storage after all. If successfully commercialized, the unusual sulfur chemistry battery could reshape multiple industries at once. Electric vehicles could travel much farther, renewable grids could store energy for longer periods, and aircraft could finally become electric for regional routes.
- More importantly, the technology shows a shift in thinking. Instead of replacing lithium completely, scientists are combining lithium’s reliability with sulfur’s enormous energy capacity. That combination could push battery performance forward again after years of incremental improvements.
- The modern world depends on batteries more than most people realize. Every step toward better storage brings cleaner transportation, more reliable renewable power, and more capable electronics. While commercialization will still take time, this sulfur chemistry breakthrough may mark the beginning of the next generation of energy storage — one that finally matches the scale of our future energy needs.
FAQs on Unusual Sulfur Chemistry
1. What Is an Unusual Sulfur Chemistry Battery?
It is an advanced lithium-sulfur battery that stabilizes sulfur reactions using a structured host material, preventing degradation and allowing much higher energy storage.
2. How Does It Compare to Lithium-Ion Batteries?
Lithium-ion batteries are reliable but limited in energy density. Sulfur-based batteries can store significantly more energy per weight, potentially enabling longer driving ranges and lighter devices.
3. When Could It Reach the Market?
Researchers are still testing durability and scaling production. Commercial use may appear later this decade if development continues successfully.
4. Is It Safer Than Current Batteries?
The controlled reactions reduce overheating risk, which may lower the chance of battery fires, though full safety certification is still required.